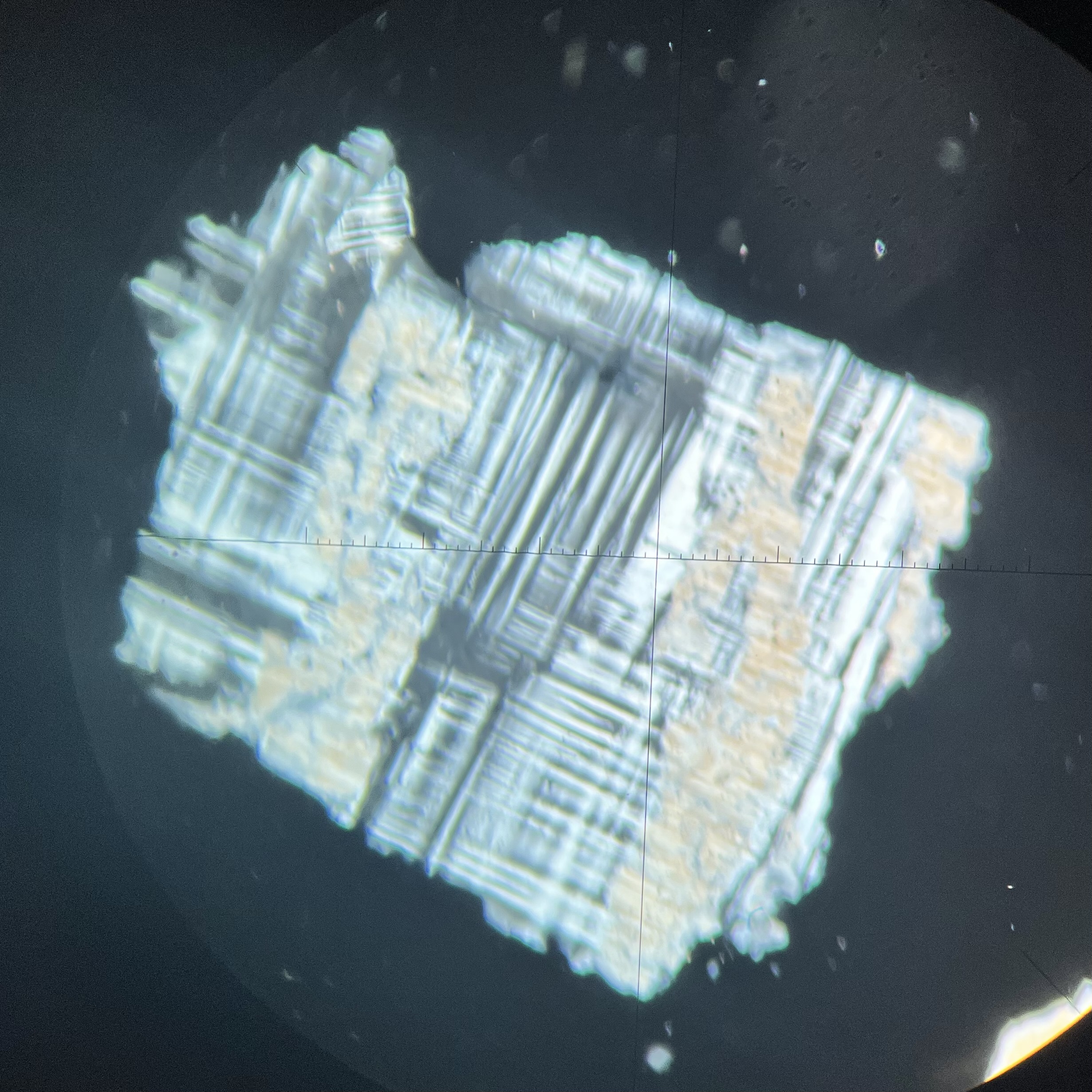
alison sent me the wikipedia article for hot-dip galvanization (https://en.m.wikipedia.org/wiki/Hot-dip_galvanization), or the process of coating iron or steel in zinc to form ZnCO3 (zinc carbonate) to prevent rusting. it forms an easily recognizable spangled pattern which immediately caught my attention as it looks crystalline! after talking to my dad (a geochemistry professor) i discovered that the formation of the spangled pattern is a matter of grain boundary energies (https://en.m.wikipedia.org/wiki/Grain_boundary), where each crystal attempts to minimize boundary energy as it grows, resulting in the shape characteristic of the crystal (when other crystals don’t prevent that shape from forming). this made it clear that a specific mineral is resulting from the galvanization process: smithsonite! (https://www.mindat.org/min-3688.html) essentially, this mineral doesn’t have time to grow thick, so it creates a thin, rust-resistant coat; but as it grows, it leaves behind the pattern typical of hot-dip galvanization.
this year’s mincup winner was rhodochrosite, a reddish manganese carbonate commonly found near silver veins. i was looking up more about it and discovered it’s actually been found as a biomineral formed by some fungi! i’m super into both fungi and minerals, so i’m looking into this a bit more. here’s a cool paper i found: https://www.mdpi.com/1422-0067/24/23/17093